The German-speaking SAP user group DSAG has released its annual investment report. The good news: Like almost every year, the majority of DSAG members are increasing IT as well as SAP budgets.
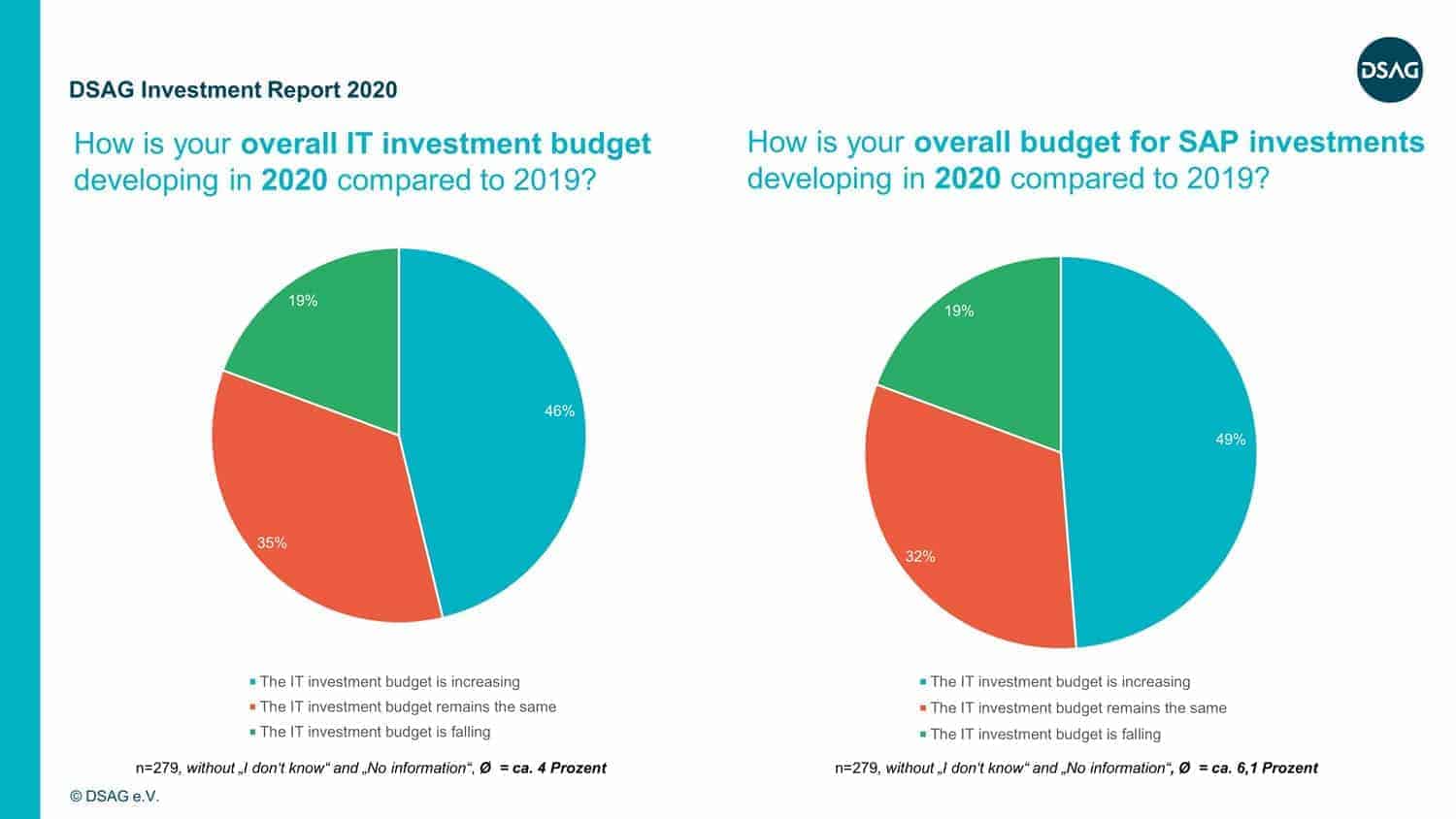
“The generally high level of readiness to invest is particularly noteworthy given the uncertain economic environment. However, this is not the same across all sectors. In manufacturing, 24 percent of respondents said their IT budgets had fallen. This could be due to the fact that, as a result of the current economic development, companies such as those in the automotive sector remain somewhat cautious about the future despite digitalization,” says Dr. Marco Lenck, DSAG Chairman, analyzing the numbers.
The bad news: Just because IT budgets are increasing, it doesn’t necessarily have to mean that companies are investing in innovative technologies.
What DSAG’s report fails to mention
An E-3 analysis of SAP’s current price and conditions list shows why SAP customers have to increase their budgets: the functionality of many SAP software components has gradually and persistently been reduced. For example, NetWeaver Engines, once capable of many functions, are now specialized on solving individual tasks.
This specialization of SAP software components means customers now need more of them to achieve the same functional scope they had before. More SAP software means more licenses, more licenses mean more fees and more fees mean more investments.
More financial resources allocated to SAP software therefore doesn’t have to mean that companies are investing more in innovative technologies like IoT, AI or even SAP Leonardo. Most of the budget probably goes to the steadily increasing costs of keeping SAP systems operational. Additionally, SAP consulting is getting more expensive due to the upcoming migration to S/4 Hana. Until 2027, all customers should migrate to SAP’s new software generation but already there’s a shortage of skilled experts and external consultants. Consequently, companies need to invest in in-house know-how and training, causing IT and SAP budgets to increase even further.
In some cases, SAP customers will find that new, higher prices for SAP software don’t apply to them. This is due to older SAP licensing contracts pertaining to NetWeaver Engines and Professional Users, for example. These licenses are valid and will continue to be as long as SAP customers do not agree to new general terms and conditions.
Leveraging older licenses and comprehensive license management, customers can reduce and avoid high IT costs and use their increased SAP budgets for things that matter in the digital age.





















Add Comment